|
Polyester Resin |
|
Polyester resin is normally polymerized by using a catalyst. The most common catalysts are liquids which contain an organic peroxide and other materials. The rate of polymerization or also known as the gel time, pot life, etc... is a function of various parameters including:
I am running some tests to start characterizing the polymerization of polyester resins to help provide some tools for mixing and working with this resin. The test is performed by mixing a known mass of resin with a controlled amount of catalyst at a specific temperature. The relative amount of polymerization is observed versus time and then plotted. The intent will be that this will create a family of curves with which one can use to estimate the gel time or pot life of a particular resin mix at a known temperature. The hardness of the curing resin is qualitatively scaled from 0 to 10 based on these observable features
Using this scale, one would be able to work with the material while it was less than 4, preferably 0 to 2. When the material reached 4, you would sense that it is about to gel. It is "going off". Resin System A
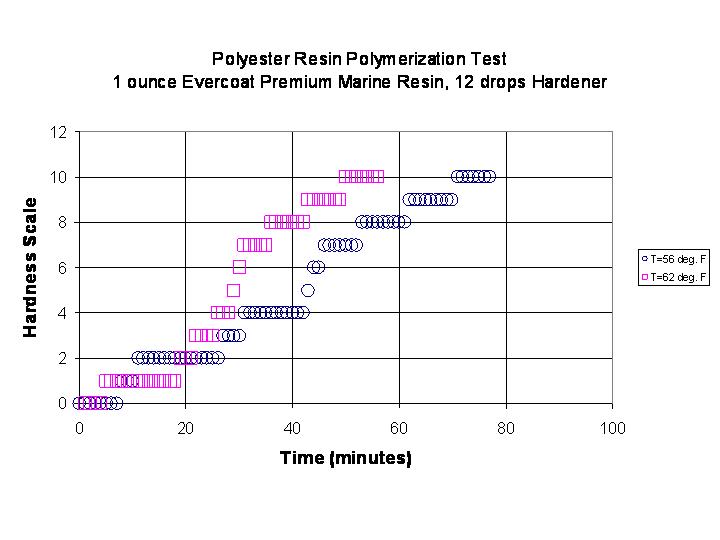
The manufacturer of this resin claims a working time of 10-12 minutes before gelling at a temperature of 71 to 90 degrees F. Below 60 degrees F, the resin may not cure. The results of two tests are shown in the graph below.
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
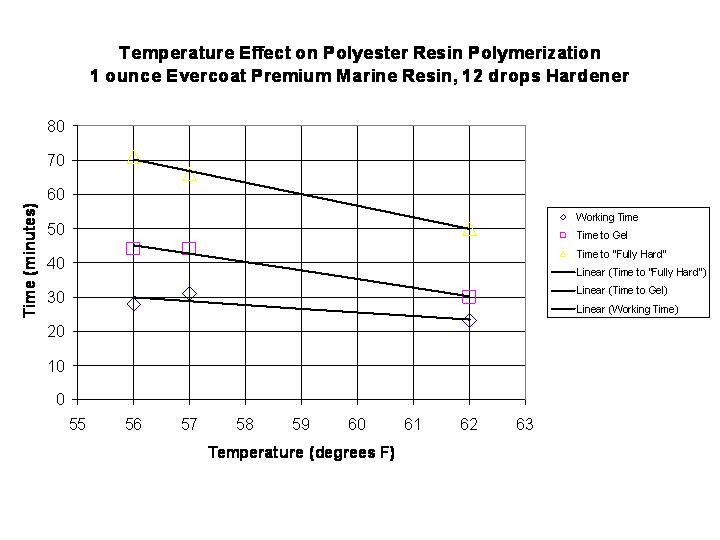
One sees that with this resin/catalyst system at 62 degrees F, the useful working time is about 23 minutes and at 56 degrees F the useful working time is extended approximately 5 minutes to 28 minutes. The gelling event which is seen as an inflection point is also delayed by about 14 minutes due to the change in temperature. A graph of all of the current data for 12 drops of hardener is shown in the graph below:
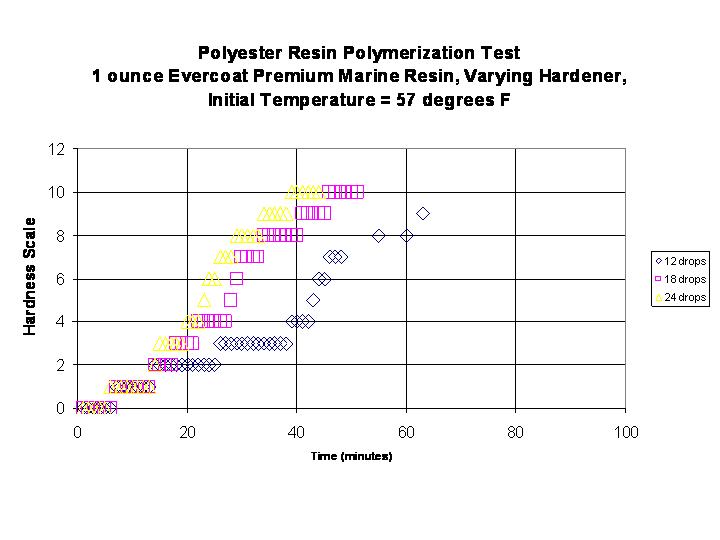
A variation of this system is to change the amount of hardener at a constant temperature. Three tests at the same initial temperature of 57 degrees F were run with 12, 18, and 24 drops of hardener. The results from this test are shown below:
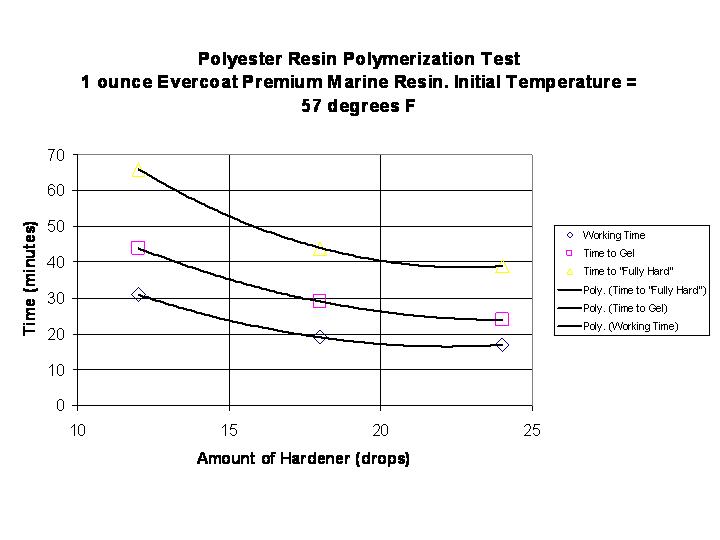
One sees that the amount of catalyst noticeably changes the rate of polymerization and it appears to be non-linear. Another way to look at this data is to graph the various "times" versus the amount of hardener. This information is in the graph below:
|